Hydrocortisone and Acetic Acid: Package Insert / Prescribing Info
Package insert / product label
Dosage form: otic solution
Drug class: Otic steroids with anti-infectives
Medically reviewed by Drugs.com. Last updated on Apr 29, 2025.
On This Page
Hydrocortisone and Acetic Acid Description
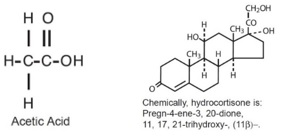
DESCRIPTION:Hydrocortisone and Acetic Acid Otic Solution, USP is a solution containing hydrocortisone (1%) and acetic acid (2%), in a propylene glycol vehicle containing benzethonium chloride (0.02%), citric acid (0.05%), propylene glycol diacetate (3%) and sodium acetate (0.015%). The empirical formulas for acetic acid and hydrocortisone are CH3COOH, and C21H30O5, with a molecular weight of 60.05 and 362.46, respectively. The structural formulas are:
Hydrocortisone and Acetic Acid is available as a nonaqueous otic solution buffered at pH 3 for use in the external ear canal.
Hydrocortisone and Acetic Acid - Clinical Pharmacology
CLINICAL PHARMACOLOGY:Acetic acid is antibacterial and antifungal; hydrocortisone is antiinflammatory, antiallergic and antipruritic; propylene glycol is hydrophilic and provides a low surface tension; benzethonium chloride is a surface active agent that promotes contact of the solution with tissues.
Indications and Usage for Hydrocortisone and Acetic Acid
INDICATIONS AND USAGE:For the treatment of superficial infections of the external auditory canal caused by organisms susceptible to the action of the antimicrobial, complicated by inflammation.
Contraindications
CONTRAINDICATIONS:Hypersensitivity to Hydrocortisone and Acetic Acid or any of the ingredients; herpes simplex, vaccinia and varicella. Perforated tympanic membrane is considered a contraindication to the use of any medication in the external ear canal.
Precautions
PRECAUTIONS: Transient stinging or burning may be noted occasionally when the solution is first instilled into the acutely inflamed ear.
Adverse Reactions/Side Effects
ADVERSE REACTIONS:Stinging or burning may be noted occasionally; local irritation has occurred very rarely.
To report SUSPECTED ADVERSE REACTIONS, contact Saptalis Pharmaceuticals, LLC at 1-833-727-8254 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Hydrocortisone and Acetic Acid Dosage and Administration
DOSAGE AND ADMINISTRATION:Carefully remove all cerumen and debris to allow Hydrocortisone and Acetic Acid to contact infected surfaces directly. To promote continuous contact, insert a wick of cotton saturated with Hydrocortisone and Acetic Acid into the ear canal; the wick may also be saturated after insertion. Instruct the patient to keep the wick in for at least 24 hours and to keep it moist by adding 3 to 5 drops of Hydrocortisone and Acetic Acid every 4 to 6 hours. The wick may be removed after 24 hours but the patient should continue to instill 5 drops of Hydrocortisone and Acetic Acid 3 or 4 times daily thereafter, for as long as indicated. In pediatric patients, 3 to 4 drops may be sufficient due to the smaller capacity of the ear canal.
How is Hydrocortisone and Acetic Acid supplied
HOW SUPPLIED:Hydrocortisone and Acetic Acid Otic Solution, USP, containing hydrocortisone (1%) and acetic acid (2%), is available in 10 mL, measured-drop, safety-tip plastic bottles (NDC 71656-064-10).
STORAGE:Store at room temperature, 20°C to 25°C (68°F to 77°F). Keep container tightly closed.
Rx only
Distributed by:
Saptalis Pharmaceuticals, LLC.
Hauppauge, NY 11788
MADE IN USA
May 2024-R3
PPM-0082
| HYDROCORTISONE AND ACETIC ACID
hydrocortisone and acetic acid otic solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Saptalis Pharmaceuticals, LLC (080145868) |
| Registrant - Saptalis Pharmaceuticals, LLC (080145868) |
More about acetic acid / hydrocortisone otic
- Compare alternatives
- Pricing & coupons
- Reviews (7)
- Side effects
- Drug class: otic steroids with anti-infectives
- En español
Patient resources
- Acetic acid and hydrocortisone otic drug information
- Hydrocortisone and acetic acid (Advanced Reading)